Our Research

Haematopoietic Stem Cells
Our translational research involves interrogating signalling events critical to the development and maintenance of both normal haematopoietic and leukaemic stem cells (HSCs and LSCs). To accomplish this, we integrate biochemical and molecular biological techniques, primary human tissue, nanoparticle characterization, chromatography, mass spectrometry, RNA sequencing, microscopy, flow cytometry, machine learning and network analyses.
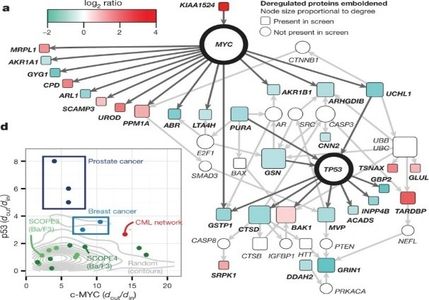
Chronic Myeloid Leukemia
Chronic Myeloid Leukaemia (CML) is a HSC disease. The hallmark of CML is the BCR-ABL1 oncogene, which encodes a constitutively active tyrosine kinase. Since 2001, the introduction of tyrosine kinase inhibitors (TKIs) has revolutionized CML treatment, but most patients must take these inhibitors indefinitely thus, TKIs do not provide a cure. TKI resistance or disease progression is primarily due to the inability to eradicate LSCs. To address this problem, we have used computational network strategies to identify signalling pathways critical to the LSC (Abraham et. al. Nature 534, 341-346, 2016). These findings contradicted the well-accepted paradigm that killing CML cells requires strategies to abrogate BCR-ABL signalling, the key defining oncoprotein of CML. This work involved: (i) generating large primary cell data sets (ii) using systems biology and computational and network analyses and (iii) validating key (drug target) regulatory hubs in CML using primary stem cells in in vitro and in vivo assays. Our work is being carried forward by Cancer Research UK into Phase II clinical trials for patients with TKI resistant CML in chronic or accelerated phase.

Aging, Stem Cells and Extracellular Vesicles
Aging can be defined as a time-dependent physiological decline that slowly impairs tissue homeostasis and leads to a block of the regeneration capacity of organs. Interestingly, as both humans and mice age there is an increase in the number of HSCs in the bone marrow, with a concurrent decrease in stem cell self-renewal capacity and reconstitution potential upon transplantation. Based on previous observations, it has been assumed that cell-intrinsic mechanisms are responsible for the decreasing functionality of HSCs, however current studies are revealing that HSCs are additionally regulated by extrinsic factors outside the cell. We investigate blood extracellular vesicles (EVs) produced at different life stages of healthy humans, to understand global signalling events that affect the health and maintenance of HSCs. Both compositional and functional characterization of circulating blood EVs will shed light into extracellular factors affecting HSCs as humans age, and processes that potentially contribute to the development of CML.

Our group was the first to identify that blood EVs are continuously maintained over adulthood and change in composition in healthy adults. Functional data support that individuals over 40 years possess EVs that stimulate HSC proliferation and potentially help support blood production as humans age. Our machine learning techniques were able to distinguish age-based EV signatures.
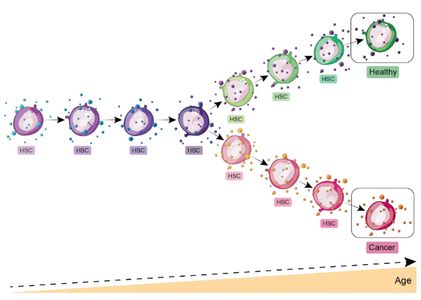
Understanding Haematopoietic Stem Cell and Leukaemic Stem Cell Maintenance During Aging
Our group is focused on understanding how HSCs are able to maintain function over time and how under certain conditions, LSC evolve. We hypothesize that not only do EVs help regulate HSCs as humans age, they will change as a person develops leukaemia. One of our group’s goals is to identify pre-leukaemic EV signatures. We want to identify malignancy at its earliest stages, targeting the cancer before it is fully established.